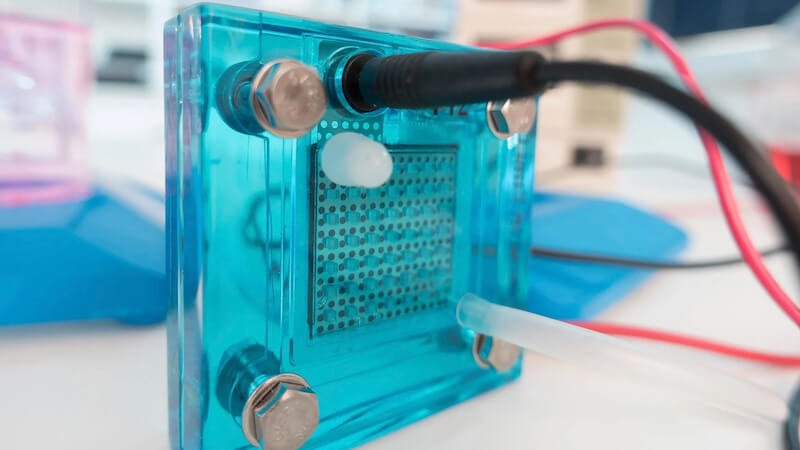
Researchers from Japan have discovered that caffeine can increase the performance of fuel cells. Because the addition reduces the amount of material used.
For many people, it is hard to imagine life without coffee and the caffeine it contains. Because it makes us awake and we feel more energetic. Researchers at Chiba University in Japan recently discovered that this doesn't just seem to apply to humans.
By adding caffeine to platinum electrodes, they were able to significantly increase the efficiency of fuel cells. The reason: The process can increase the oxygen reduction reaction and thus reduce the need for platinum. As a result, the price of fuel cells decreases with higher efficiency.
Fuel cells for a sustainable future?
The further development of fuel cells in various areas, including transportation and energy production, is considered an important step towards sustainable energy sources. The cells consist of an anode and a cathode and use an electrolyte to generate energy. They convert chemical energy from a fuel directly into electrical energy.
The researchers feed the fuel to the anode. An oxidizing agent, some oxygen from the air, is available on the cathode side. In a hydrogen fuel cell, hydrogen is oxidized at the anode. This leads to hydrogen ions and electrons. The ions move through the electrolyte to the cathode while the electrons flow through an external circuit. This generates electrical energy.
Caffeine increases fuel cell performance
The cathode combines oxygen with the hydrogen ions and electrons, resulting in water as the only byproduct. However, this affects the performance of the fuel cell. This is because the water reacts with the platinum catalyst and forms a layer of platinum hydroxide on the electrode, which leads to energy losses.
Therefore, to maintain efficient operation, fuel cells require a high platinum charge, which significantly increases costs. However, the Japanese researchers had an idea and added caffeine to the fuel cell.
The result: It increases the activity of the oxygen reduction reaction on certain platinum electrodes. This opens up the possibility of reducing platinum requirements and making fuel cells more affordable and efficient. The method apparently also has the potential to optimize the design of fuel cells and expand the area of application.
Also interesting:
Source: https://www.basicthinking.de/blog/2024/03/18/koffein-brennstoffzellen/


